A Single, Centralized Platform
One single integrated solution that connects clinical trial data by centralizing all data sources, without the complexity of using multiple separate components. Remarque Systems consolidates data, then layers on visualization and automated metrics to generate consistent, actionable data insights in real-time. By combining all your data into one powerful platform, RemarqueTM improves the operational performance of a clinical trial through the following:
- Process optimization
- Increased visibility
- Rapid communications
- Improved quality
Advanced Clinical Trial Operations
Slide the bar to see what happens when you go from disconnected data and manual processing to integrated data and automated real-time processing.
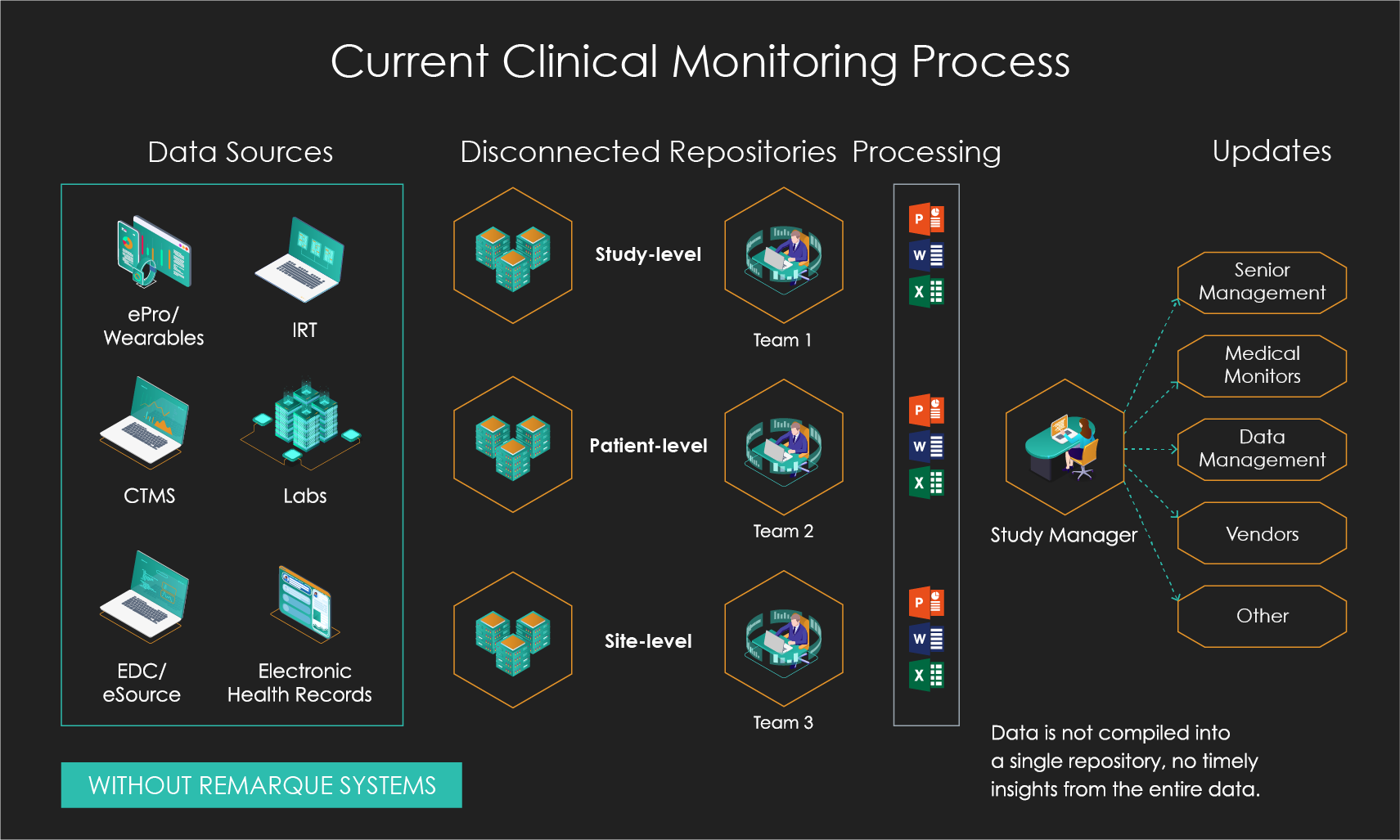
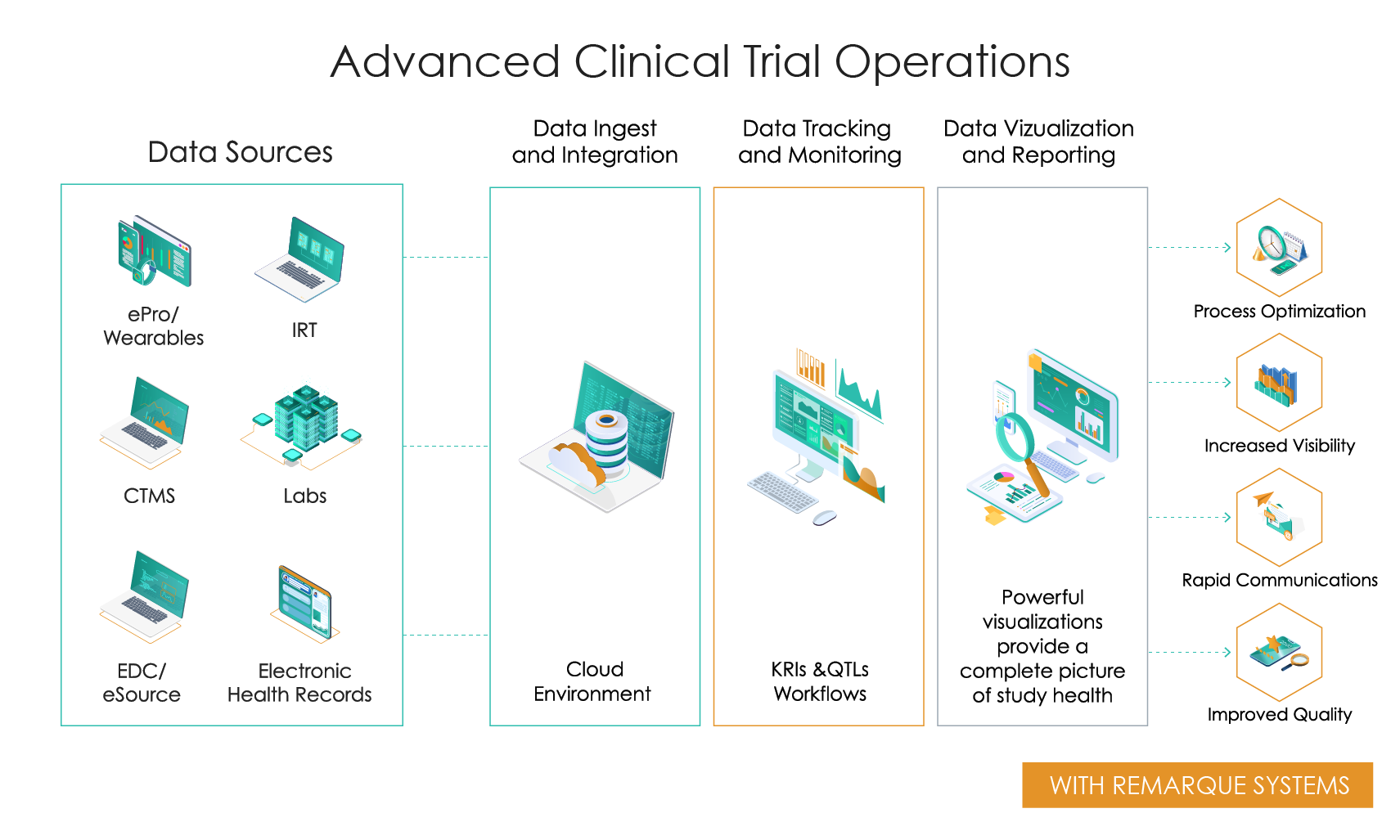
Better Insights Through Centralized Data
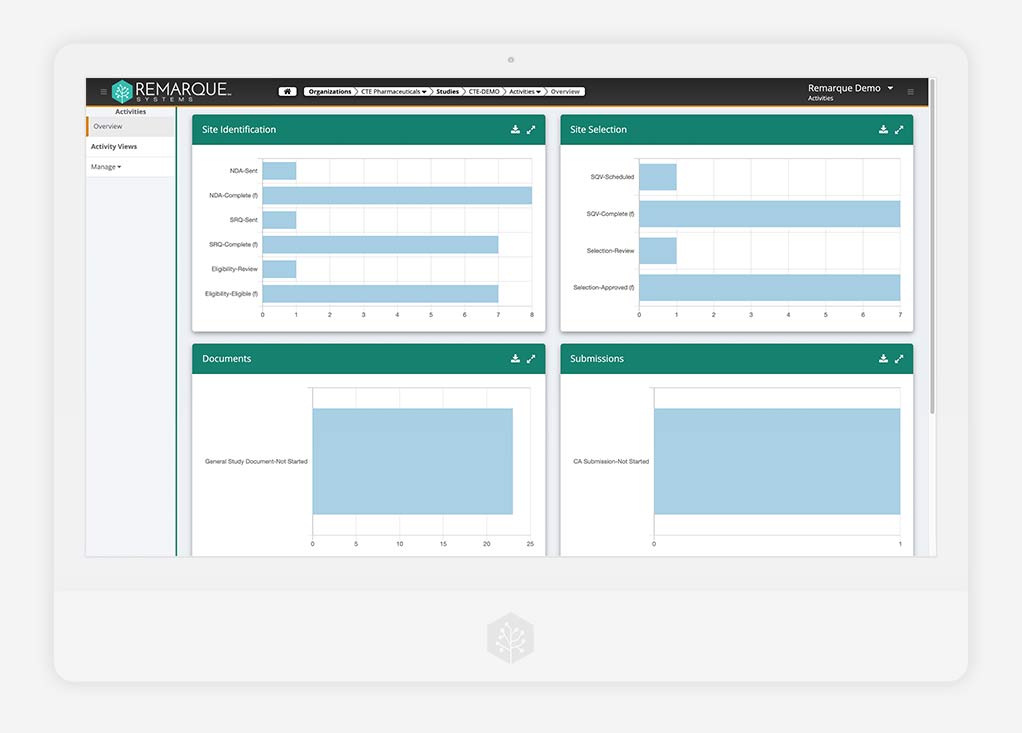
Study Start-Up
Where a successful trial launch begins its ascent
Get your study off to the right start with effective tools for every aspect of your work and data, all under one platform. Collaborate, collect, and manage with quality and control — from start-up to closure.
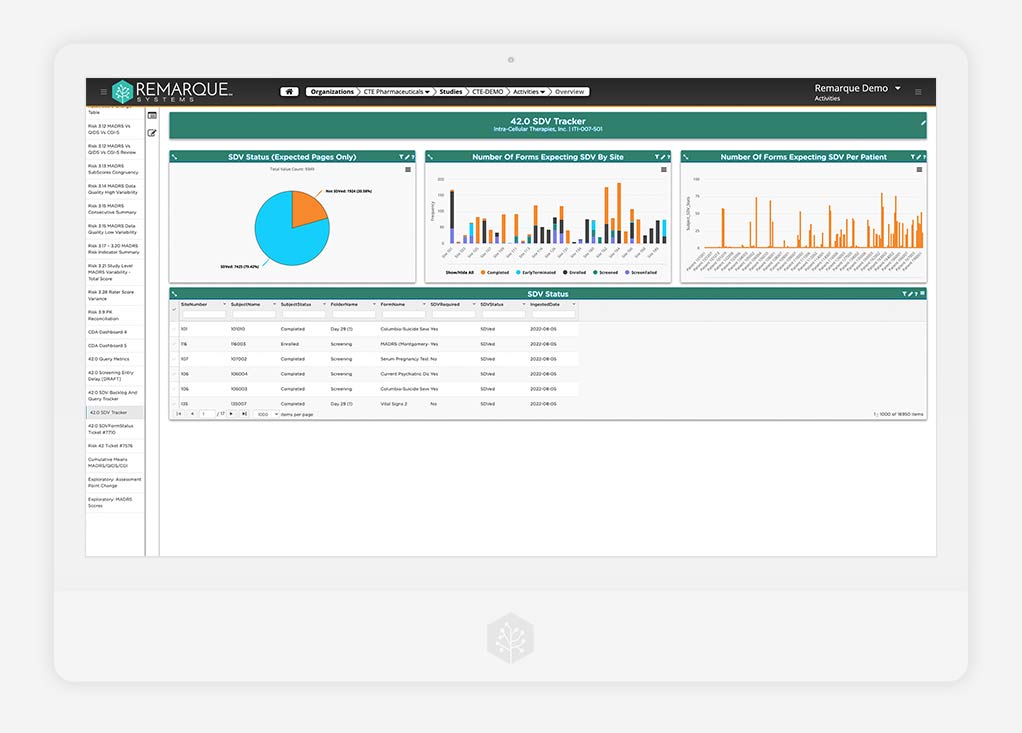
Remote Monitoring
Mitigate risks and track progress around the clock
Get 24/7 tracking of risks, study progress, and more by aggregating your study data under one powerful platform to support multiple types of monitoring including remote, central, and medical.
Risk-Based Quality Management (RBQM)
Advanced risk management
Better control your risk by filtering all study data through a single, easy-to-use platform. Quickly collaborate with your team on risk mitigation and compliance measures — ending error-prone processes and giving you the total visibility you need to react swiftly and effectively.
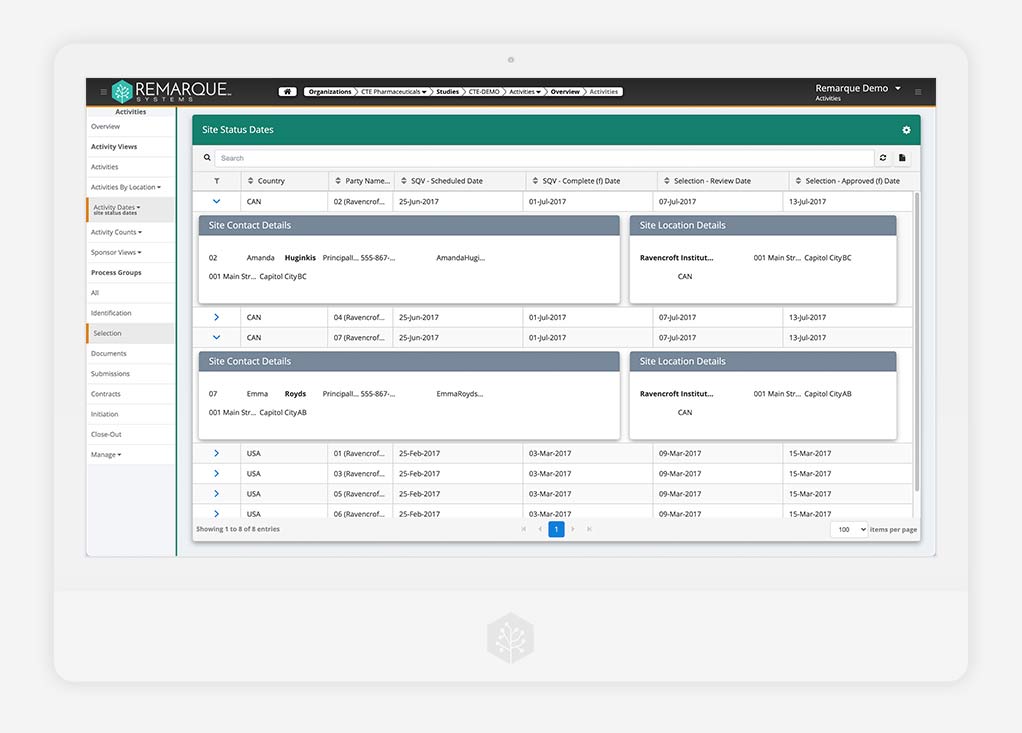
Clinical Trial Management System (CTMS)
Configurable role-based CTMS
Connect all site documentation and study data to one centralized platform. Increase productivity and collaboration. Ensure important milestones are met.
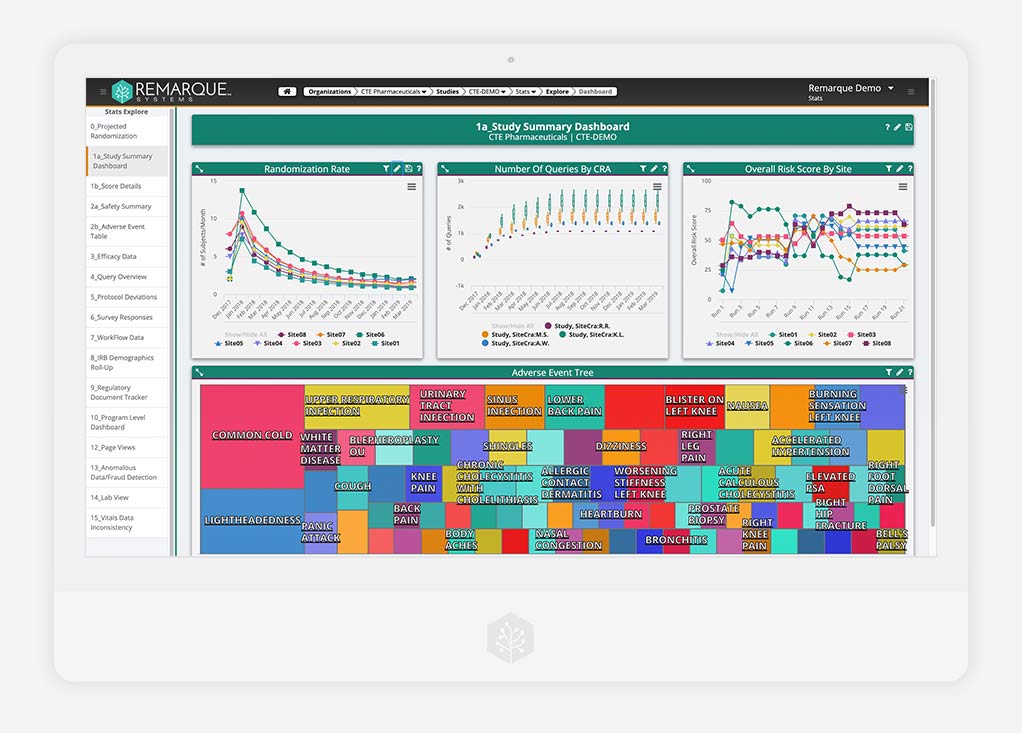
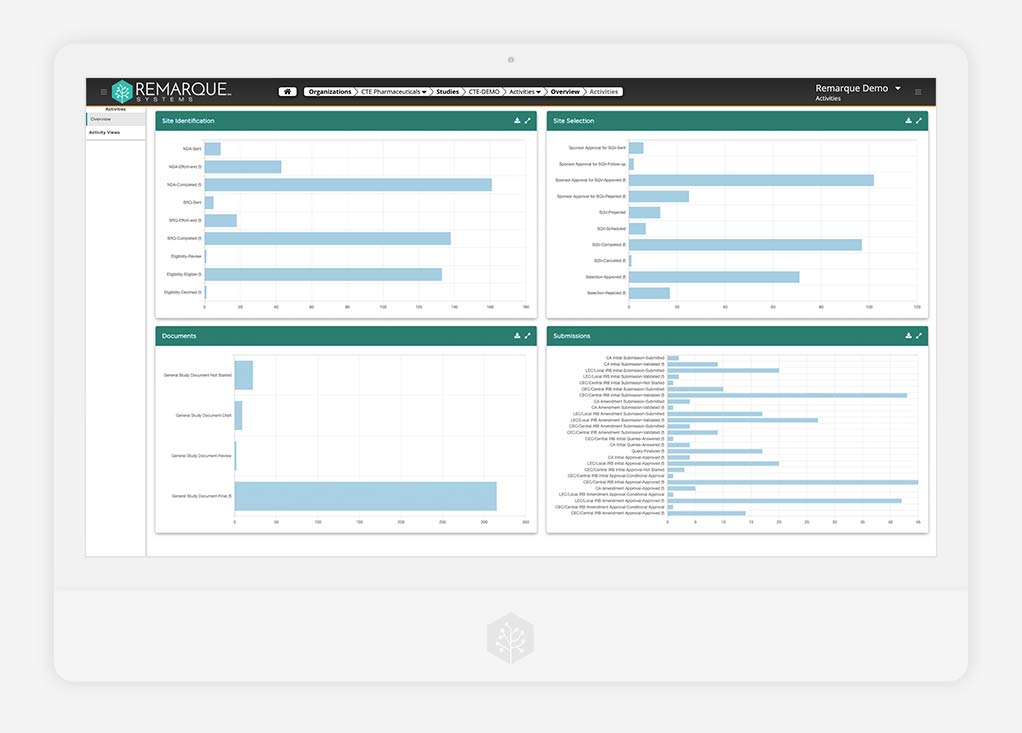
Data Analytics
End-to-end visibility and oversight in real time
Discover clear, data-driven insights in real-time. Choose the best course of action by transforming the flow of all your study data into actionable analytics, reports, and dashboards.
Services
Strategic Consulting
- Process design(QbD) & risk assessment
- Template strategy, creation & configuration
- Premium support
RBQM Consulting
- RBQM 101
- RBQM/Remarque Comprehensive Intelligent Monitoring Training (CIMT)
- ICH E6 implementation
- Success criteria evaluation
Analytics Consulting
- ADFD
- Advanced analytics application / implementation
Knowledge Transfer
- CRO-enablement
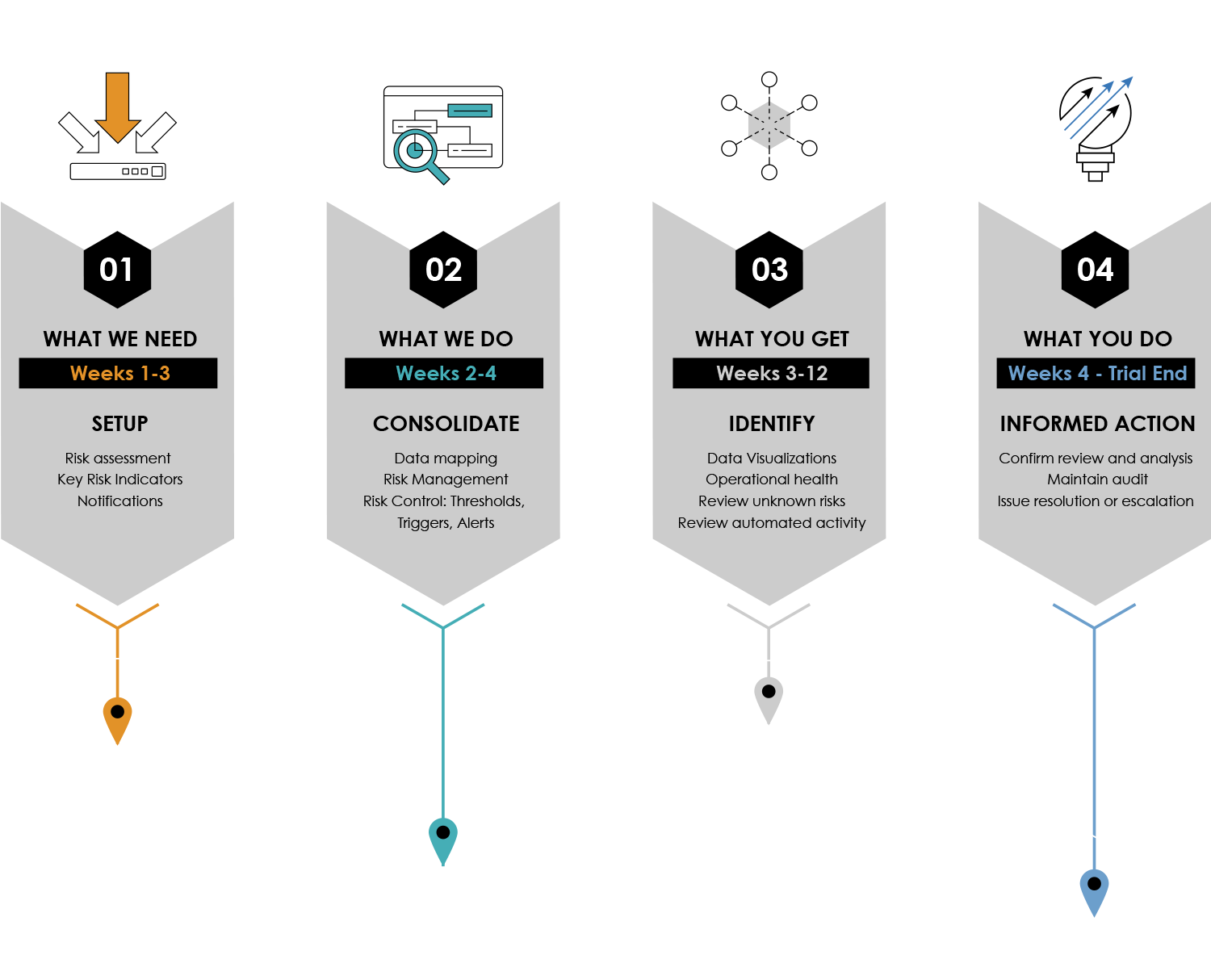
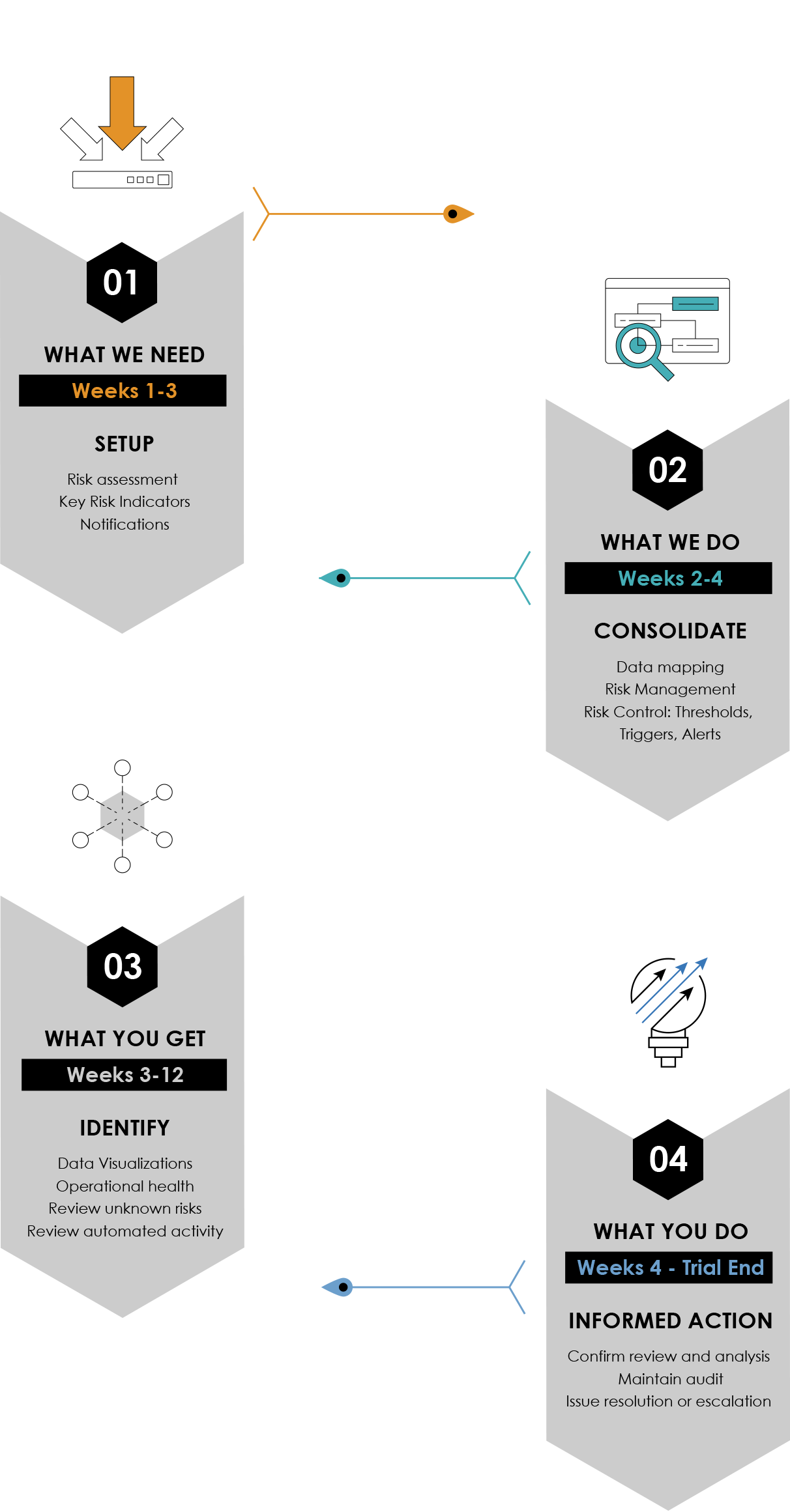
The Remarque System 4 Step Turnkey Process to Study Set-up
From design To reporting, RemarqueTM manages your trial
Fits Seamlessly Into
Your IT Ecosystem
Seamless Setup
RemarqueTM adds a layer on top of your existing technology systems, ensuring easy adaptability to your current IT environment and minimizing the need for costly change management.
Built to Evolve
Scalable and source-agnostic, RemarqueTM can be customized to any trial specifications and sizes.
Deep Insights
Role-based views into patient safety and drug efficacy, ensure team members can access the data they need in near real-time to do their jobs most effectively.
Prioritized Workflow
Protocol-based triggers and alerts clearly designate next steps, increasing patient safety and data quality, while creating a full auto-generated audit trail.
Smarter Analytics
In-process analytics and machine learning deliver effective risk prediction, detection, analysis, and management, using parameters tailored to each trial’s objectives.