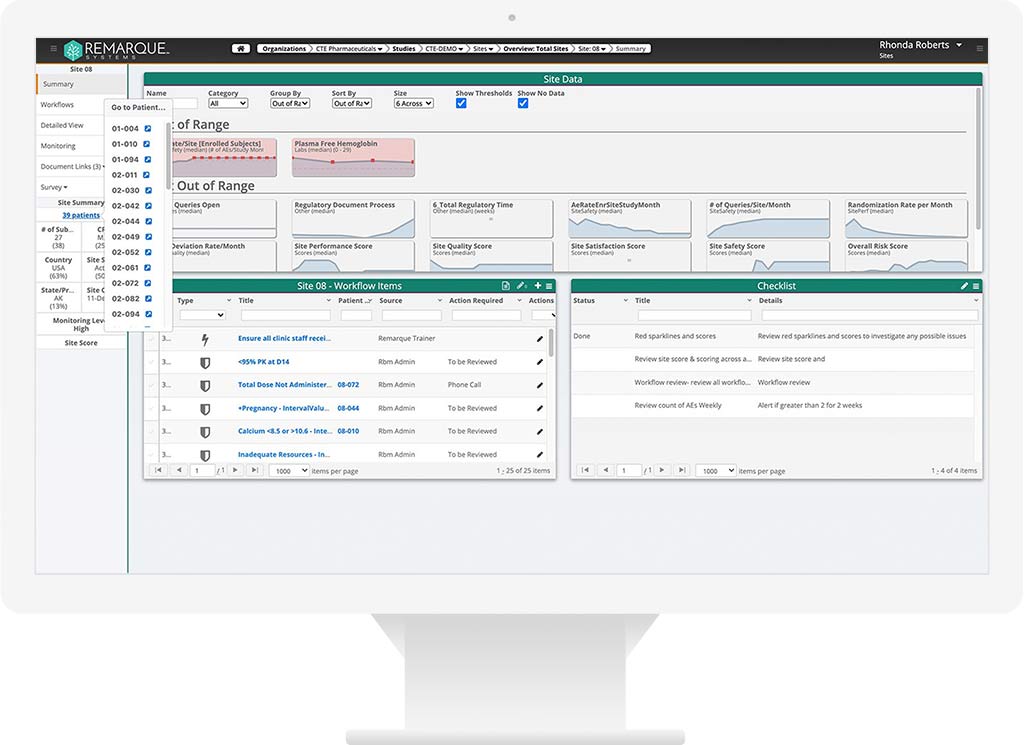
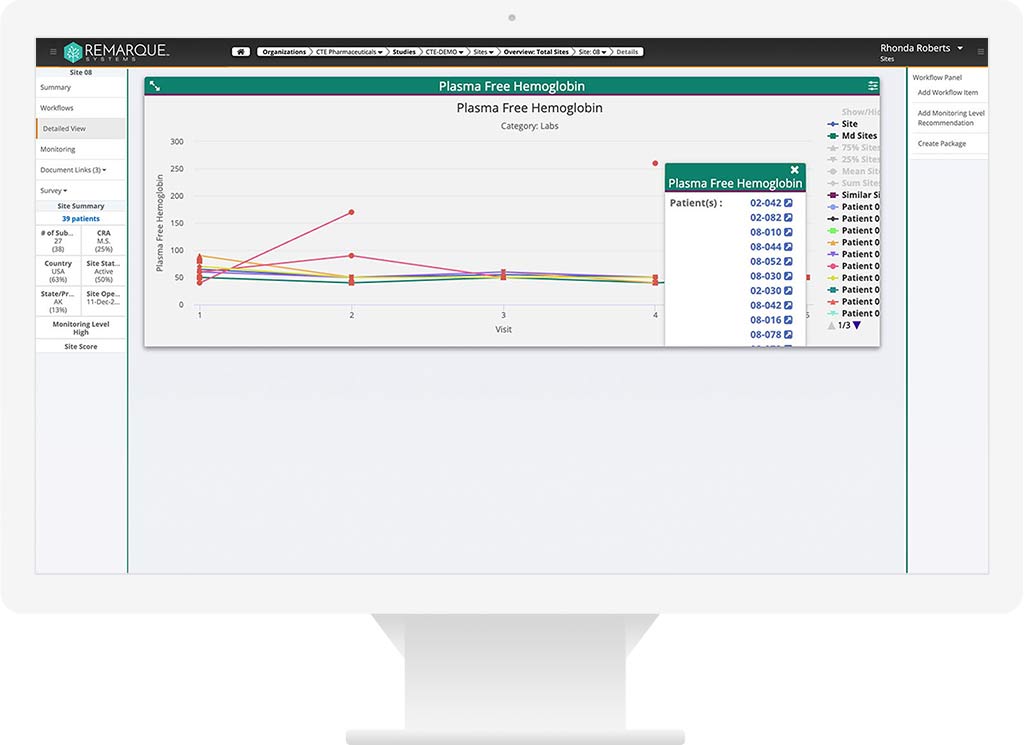
Consolidated Data.
Identified Risk.
Informed Action.
By bringing all your trial data together in one location RemarqueTM supports process optimization, increased visibility, and improved quality to your clinical trial data and operations.
Consolidate
Seamlessly bring your data together from disparate systems into a single location to gain better visibility and oversight so you can take informed actions.
Quickly identify known and unknown risks with trial-specific triggers — data outliers, adverse events, enrollment issues — in real-time. Better manage risks with greater oversight, collaboration, and visibility.
Get real-time oversight and end-to-end visibility over all your clinical trial data. Streamline your operations with clear, conclusive analytics and reports based on AI-powered data-driven insights.
The Result
All clinical trial data flows through one powerful platform — empowering increased visibility, better insights, and more informed actions.
Organizations around the world use RemarqueTM to gain better clinical trial data insights.
Our Solution
Consolidating all clinical trial data under one powerful platform delivers higher quality data, deeper insights, and clear decision paths.
Our Platform
Vendor neutral data aggregation with no coding required. Fully supports next generation trials including DCT’s and is designed to create and maintain historical knowledge.
Our Promise
RemarqueTM fits into any ecosystem supporting any type of trial. We adapt to changing clinical trial design, changing types of data, evolving regulatory demands – helping you optimize your process and minimize risk along the way
Case Study
Our strategy enabled the client to identify specific patients at risk of discontinuation—and information on why each of those patients is at risk. Our client was then able to engage in targeted activities to optimize retention.
Case Study
By centralizing the data on the Remarque platform then applying an RBM strategy, the CRO streamlined SDV while swiftly identifying anomalies. The CRO was able to roll out the analytics plan to their study team—and ultimately, to the sponsor. Ultimately, they could save time, reduce costs, and minize risk both for the sponsor and for the patients.
Case Study
“A nimble company with a focus on customer service, Remarque spent the time to brainstorm various configurations to find the optimal solution for their client, then was able to offer end-to-end deployment that noticeably abbreviated the implementation timeline. In short: They were able to launch the study in record time, while maintaining the safety of the trial team.”
Case Study
“We knew that implementing Remarque technology would improve our efficiency, but it was impossible to understand how dramatically our efficiency would increase until we actually implemented the technology and experienced the difference. We now do in 30 minutes what used to take a full 8-hour day.”