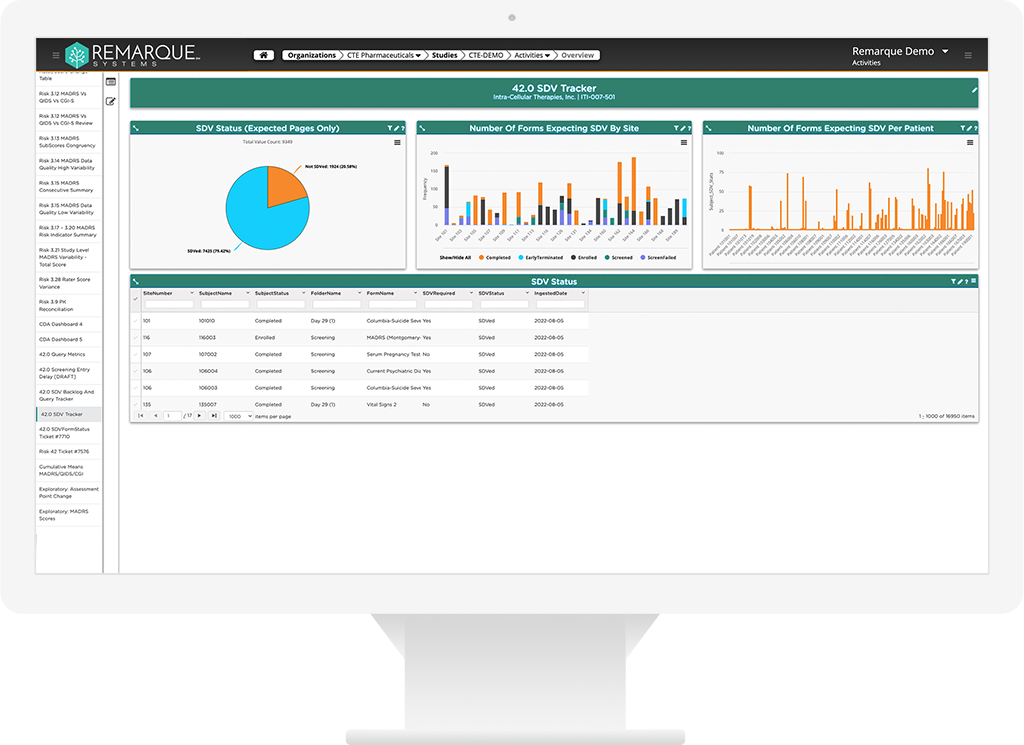
Watch the pulse of your clinical trial R&D
An integrated and validated approach to all types of monitoring including, on-site, remote, and centralized. Real-time risk mitigation and management of site visits, protocol deviations, communication logs and patient profiles.
Benefits
- Increased end to end visibility with centralized data monitoring capabilities
- Instant insight with signal alerts and visualizations
- Increased efficiency with fully integrated workflows and checklists to standardize and guide monitoring reviews
- Supports a reduction in on-site monitoring activities
Features
- Aggregates data across all source systems into one single enviroment
- Rapid signal dectection
- Patient profiles to support eligibility reviews, safety reviews, etc
- Longitudial data reviews and visulizations broken down to the study patient and site level
Configurable Applications
RTM + CTMS
Easily execute onsite, remote, and centralized monitoring visits in a single intuitive platform with consistent and complete reporting system.
RTM + RBQM
Take your real time monitoring to the next level by adding RBQM to better manage risk and demonstrate oversight.
RTM + RBQM + CTMS
Manage all types of monitoring and the associated activities in one centralized platform to drive quality and consistency in execution.