Introduction
Infused into virtually everything we touch, technology can feel like both a blessing and a curse. While some technologies are easy to use right out of the box, others require set-up in order to be useful. At times, that set-up and even the eventual use of the technology can feel daunting and frustrating. However, in most cases, once the hurdles have been overcome, value can be found in the technology.
As clinical trials become more complex, risk-based monitoring (RBM) technologies have become more sophisticated. For sponsors, unlocking the full value of an RBM technology requires proactive planning, thoughtful implementation and ongoing strategic execution following deployment.
In this white paper, we discuss how to build a foundation prior to technology implementation. We also explore key factors to consider during implementation and strategies for operational execution after implementation.
Defining RBM Technology
Many people associate risk-based monitoring with efforts to reduce source document verification (SDV) or decrease on-site monitoring visits. However, in reality, risk-based monitoring is part of a much broader concept called risk-based quality management, which is a systematic process put in place to identify, review, assess, control, and communicate the risks associated with a clinical trial.
Within this larger context, RBM technology refers to software that:
- Provides visibility into an entire study, allowing stakeholders to manage and mitigate risks so that the study stays on track
- Enables consistent communication across the entire team
- Provides ongoing oversight of patients, sites and the study as a whole to facilitate decision-making (e.g., percentage of SDV required, frequency of on-site monitoring visits, safety, site performance, etc.)
It is important to keep in mind that different organizations and even individual end users within a single organization may have different motivations and goals for utilizing RBM technology, which can lead to conflicting priorities that must be carefully considered during technology implementation.
The RBM Implementation Process
Implementation of an RBM technology involves three primary steps:
- Data Input. Data comes into the technology platform, ideally from any source.
- Data Analysis. The technology platform aggregates and analyzes the data.
- Data Output. The result of this analysis is visualizations and/or actionable outputs which are made available to the end user(s), typically the sponsor or contract research organization (CRO) personnel. Ideally, the end user(s) would be able to view patient-, site-, and study-level information, as well as data required for oversight.
Components of Successful Implementation
The three foundational components for successful implementation of RBM technology are people, processes, and technology. Taking all three components into consideration and planning ahead can help minimize frustration and save time, ultimately leading to a smooth, successful implementation.
The Right People
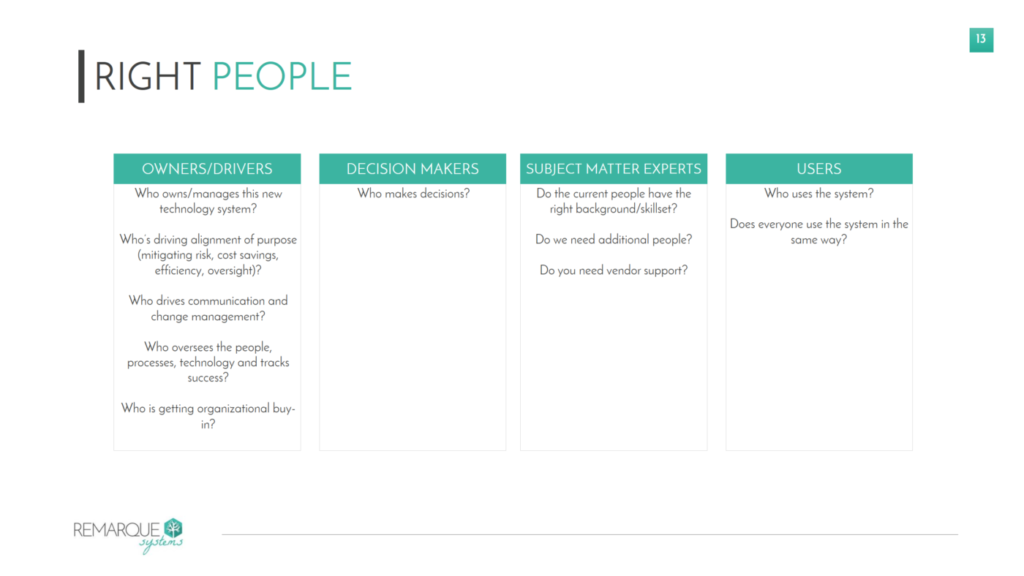
The people—or key stakeholders—will come from multiple departments within an organization, from clinical operations, data management, and safety to IT, training, statistics, and regulatory. Each of these stakeholders provides value in different ways during implementation. To build the right team, it may be useful to categorize stakeholders into four buckets (see Figure 1):
- Owner/drivers. These are the people who will own and drive the implementation process.
- Decision makers. These are the stakeholders who will make decisions on how the RBM technology is implemented.
- Subject matter experts. These are the people who have the right background and skillset to advise on the implementation that makes the most sense for a particular study.
- These are the stakeholders who will be utilizing the RBM technology during the course of a clinical trial. Considering the needs and requirements of end users in the early stages of implementation ensures that the system is being set up in a way that is most useful, efficient, and cost-effective post-implementation.
From a ‘people’ perspective, most of the issues encountered during implementation stem from lack of internal alignment and buy-in. Lack of a well-defined purpose and roles can lead to delays in the implementation process, inefficiency during and after implementation, lack of clarity or direction, and failure to gain the necessary traction. It is critical for all stakeholders involved in the set-up and use of the system to understand why and how the technology will be used, so they will be invested in contributing to successful implementation.
Figure 1. Building the Right Team for Successful Implementation
The Right Process
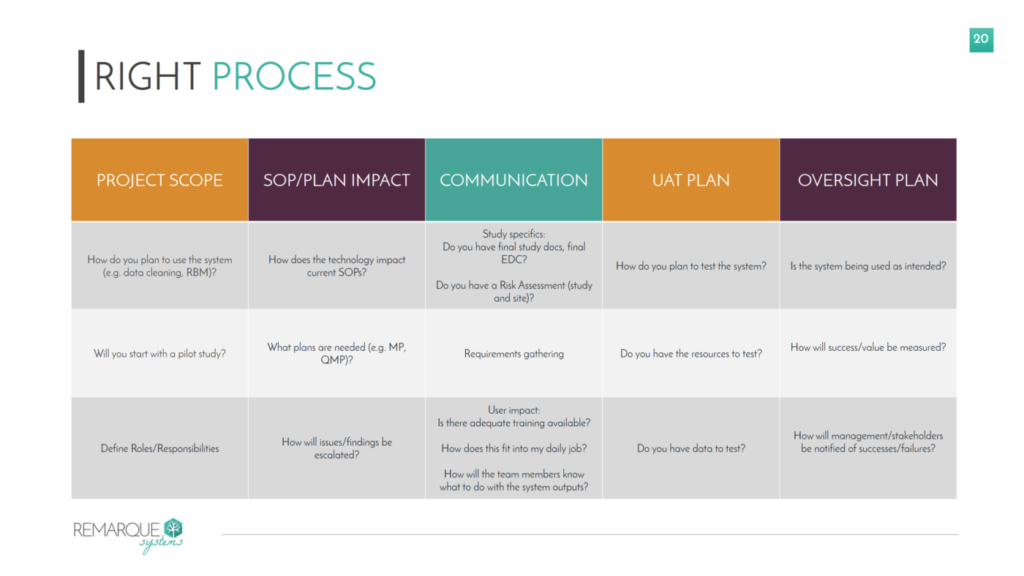
The process of implementation involves requirements gathering, planning, and testing, and should cover five areas (see Figure 2):
- Project Scope. The scope should be aligned with the needs of the end user(s) and should include clear definitions of roles and responsibilities. Most sponsors benefit from starting with a pilot study in the technology, which allows them to familiarize themselves with the implementation process, test the system, and establish internal processes on a small scale rather than simultaneously jumping into implementations of multiple studies.
- SOP/Plan Impact. This involves review of existing procedures and plans, and updating them or creating new processes, as needed. This is also an opportunity to document how issues that are identified by the system are to be escalated internally.
- It is important for sponsors to define how they will communicate with both the technology vendor and the end users.
- User Acceptance Testing (UAT) Plan. Many sponsors fail to give this aspect of the process appropriate consideration. UAT can be performed at both the system level and the study-specific level. Keep in mind that the time and resources involved in UAT need to be included in the overall implementation timeline and budget.
- Oversight Plan. The oversight plan outlines how success and value will be measured, and how these metrics will be communicated to all stakeholders.
Figure 2. Developing the Right Process for RBM Technology Implementation
The Right Technology
Ultimately, the purpose of the technology is to support both people and process. For the sake of this discussion, RBM technology requirements will focus on access to and transfer of data.
Access to Data. The importance of access cannot be overestimated—organizations rarely own all of their data and virtually every RBM technology implementation involves the need to access third-party data.
It is critical to understand who controls the data and where the data is housed. It is also essential for all stakeholders, including sponsors and third-party vendors, to understand who has the authority to share data and what permissions are required for sharing, as well as the frequency and format that data is shared.
Transfer of Data. Once sponsors have established where their data is and how it can be shared, they then need to understand how that data will be transferred to or accessed by the RBM technology vendor; taking into account the specifications from the vendor(i.e. certain RBM technologies require data to be in a specific format). Sponsors should also evaluate whether they have the technical resources required for collection and transfer of data. Finally, sponsors should consider what happens if there are changes to their databases. Ideally, the plan would be to minimize changes, but there should be a process in place to notify the RBM technology vendor of planned database changes to eliminate downstream errors.
Making RBM Technology a Friend
Investing time in getting an RBM technology implementation right enables sponsors and end users to leverage that technology to make their jobs easier, facilitate communication, and see what they need to see when they need to see it. The right implementation should be flexible and configurable, so the visualizations and actionable outputs produced provide stakeholders with the information they need to make decisions. Below is a sampling of seven use cases that demonstrate how RBM technology can add value throughout study planning and conduct:
- Risk Management. The latest version of the ICH Good Clinical Practice Guidelines (ICH E6 R2) requires sponsors and CROs to develop a systematic risk-based approach to clinical trial conduct using a risk assessment. Some RBM technologies include risk assessments with full audit trials, which allows users to not only list their risk mitigations but also associate them with the data and analysis within the software.
- Issue Management. RBM technology provides notifications when issues are identified at the patient-, site- or study-level. In addition, the right technology should enable the user to update and track the issue to closure, and even allow the user to assign the issue to the person responsible for working to its resolution.
- Patient Oversight. Rather than having to navigate to multiple case report forms (CRFs) to view patient-level information, RBM technology allows clinical research associates (CRAs) and other stakeholders to see patient data at a glance, including adverse events, concomitant medications, trends and even in comparison to other patients in the study.
- RBM technology can provide visualization of safety information and adverse events of interest.
- Study Oversight. RBM software can provide insight on CRA workload, site performance and adverse events across an entire study.
- Institutional Review Board (IRB)/Ethics Committee (EC) Oversight. Some RBM technologies enable users to see IRB/EC approval information to help determine why delays are occurring at a site or to help with decision-making about repeat use of a site, IRB, or EC.
- Site Performance. The right technology should allow users to not only see a compilation of existing study data, but also gather subjective information about sites. This information can be used to compare site performance, identify issues, and focus resources on the appropriate sites so study data is minimally impacted.
Conclusion
In essence, the right RBM technology should be customizable to the unique, specific needs of a sponsor. The key is to establish the right foundation of people, processes, and technology to make RBM software your friend.