Advanced risk management
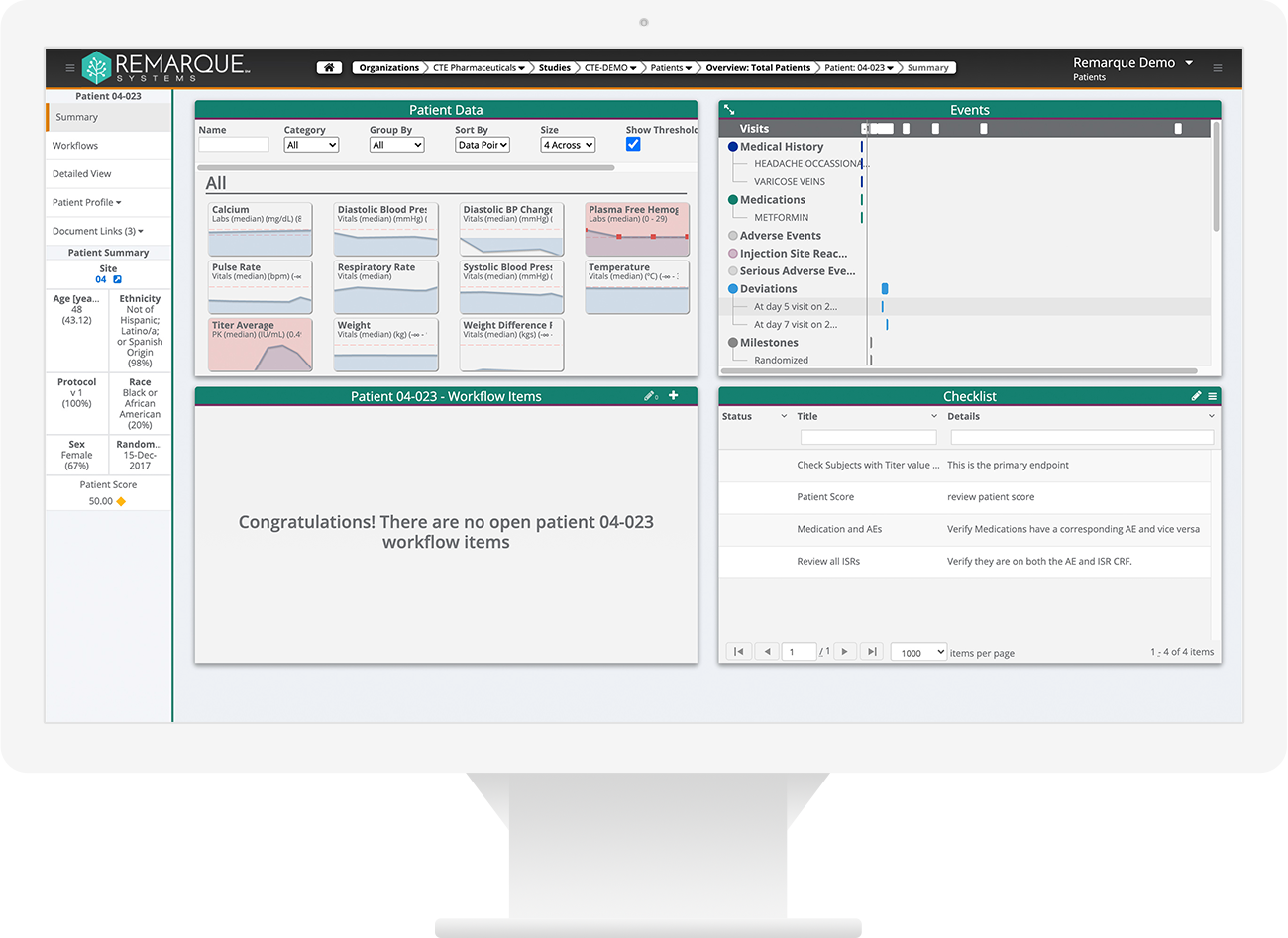
Greater oversight, visibility and collaboration. Reduce risky manual processes. Quickly identify, monitor, manage, and report on known and unknown risks. Full audit trail to demonstrate compliance with ICH E6(R2) regulations for risk management.
Benefits
- One place to document, categorize, and mitigate study risks
- Ensures study meets traceability requirements
- Transforms the way you manage study risks
- Ends error-prone manual data processes (e.g., spreadsheets)
- High-Level oversight of your portfolio – Status updates across studies
- Increased flexibility in managing risks
Features
- Identify, monitor, manage, and report on known and unknown risks
- Full audit trail to demonstrate compliance with ICH E6(R2)
- System-generated alerts
- End-to-end issue management
- Six Out of the box risk templates
Configurable Applications
RBQM + CTMS
Easily execute onsite, remote, and centralized monitoring visits in a single intuitive platform with consistent and complete reporting.
RBQM + Study Start-Up
Launch your study with data quality and risk mitigation in mind. Quickly collaborate with your team and gain the visibility you need for effective startup and quality control.
RBQM + CTMS + Study Start-Up
Take your clinical trial to the next level by adding RBQM and effectively manage your study data from the first site identification to the last patient visit.